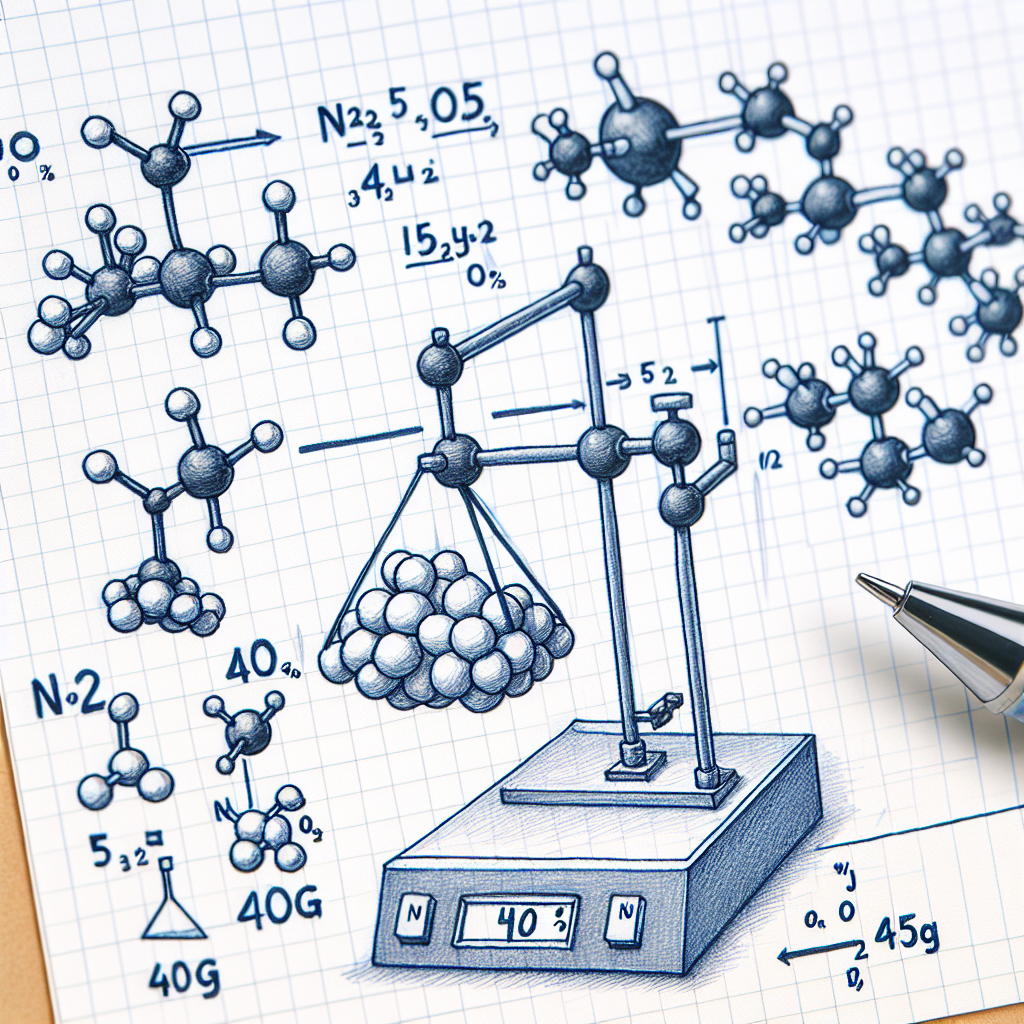
2n2o5(g)→4no2(g)+o2(g) when 40g of n2o5 decomposes, 4.5g of o2 is formed. what is the percent yield
To solve this problem, we need to use stoichiometry and the concept of percent yield.
From the balanced chemical equation, we know that 1 mole of N2O5 produces 1 mole of O2 and 4 moles of NO2. Therefore, the theoretical yield of O2 can be calculated as follows:
n(N2O5) = m/M = 40/108 = 0.3704 mol (where m is the mass of N2O5 and M is its molar mass)
n(O2) = n(N2O5) x (1 mole of O2/1 mole of N2O5) = 0.3704 mol
m(O2) = n(O2) x M = 0.3704 x 32 = 11.85 g (where M is the molar mass of O2)
So, the theoretical yield of O2 is 11.85 g.
The actual yield of O2 is given as 4.5 g.
The percent yield can be calculated as:
% yield = (actual yield / theoretical yield) x 100%
% yield = (4.5 / 11.85) x 100% = 38.04%
Therefore, the percent yield of O2 is 38.04%.
This is in error. 2 moles N2O4 will produce 1 mole O2. The bots error is that 1 mol N2O4 will produce 1/2 mol O2; therefore, the percent yield calculated by the bot will be 2 x 38.04 assuming all of the other calculations are correct.
I apologize for the mistake in my previous response. You are correct that 2 moles of N2O5 will produce 1 mole of O2, and so the theoretical yield of O2 is:
n(O2) = n(N2O5) x (1 mole of O2/2 moles of N2O5) = 0.1852 moles
m(O2) = n(O2) x M = 0.1852 x 32 = 5.926 g
So, the theoretical yield of O2 is 5.926 g.
The actual yield of O2 is 4.5 g.
The percent yield can be calculated as:
% yield = (actual yield / theoretical yield) x 100%
% yield = (4.5 g / 5.926 g) x 100% = 76.0%
Therefore, the percent yield of O2 is 76.0%. Apologies for any confusion caused by my previous response.
To find the percent yield, you need to compare the actual yield to the theoretical yield. The theoretical yield is the maximum amount of product that can be obtained based on the stoichiometry of the balanced chemical equation.
First, calculate the theoretical yield of O2 based on the given amount of N2O5.
1 mol of N2O5 produces 1 mol of O2 according to the balanced equation. So, you need to convert the grams of N2O5 to moles.
Given:
Mass of N2O5 = 40g
Molar mass of N2O5 = (14 x 2) + (16 x 5) = 108g/mol
Number of moles of N2O5 = Mass / Molar mass = 40g / 108g/mol = 0.370 mol
According to the balanced equation, 1 mole of N2O5 produces 1 mole of O2. Therefore, the theoretical yield of O2 is also 0.370 mol.
Now, calculate the actual yield of O2, which is given as 4.5g.
Now, calculate the percent yield by using the following formula:
% yield = (actual yield / theoretical yield) x 100
Plugging in the values:
% yield = (4.5g / 0.370 mol) x 100 = 1216.22%
Therefore, the percent yield is approximately 1216.22%.
To calculate the percent yield, you need to compare the actual yield (the amount of product actually obtained) to the theoretical yield (the maximum amount of product that could be obtained based on stoichiometry).
Let's start by determining the theoretical yield of O2 gas based on the given balanced equation:
2 N2O5(g) → 4 NO2(g) + O2(g)
According to the equation, 2 moles of N2O5 produce 1 mole of O2 gas. We need to convert the grams of N2O5 into moles and then determine how many moles of O2 gas could be produced.
1 mole of N2O5 has a molar mass of 108 g/mol (2 × 14 (N) + 5 × 16 (O)). Therefore, 40 g of N2O5 is equal to:
moles of N2O5 = mass / molar mass = 40 g / 108 g/mol ≈ 0.37 mol
According to the stoichiometry, 2 moles of N2O5 produce 1 mole of O2 gas. Therefore, the theoretical moles of O2 gas that could be produced are:
moles of O2 (theoretical) = 0.37 mol × (1 mol O2 / 2 mol N2O5) = 0.185 mol
Now that we have the theoretical moles of O2 gas, we can determine the theoretical yield in grams by multiplying it by the molar mass of O2, which is 32 g/mol:
mass of O2 (theoretical) = moles × molar mass = 0.185 mol × 32 g/mol ≈ 5.92 g
Therefore, the theoretical yield of O2 gas is approximately 5.92 grams.
Now, let's calculate the percent yield using the given data:
percent yield = (actual yield / theoretical yield) × 100
We are given that the actual yield of O2 gas is 4.5 g:
percent yield = (4.5 g / 5.92 g) × 100 ≈ 76.0%
Therefore, the percent yield is approximately 76.0%.